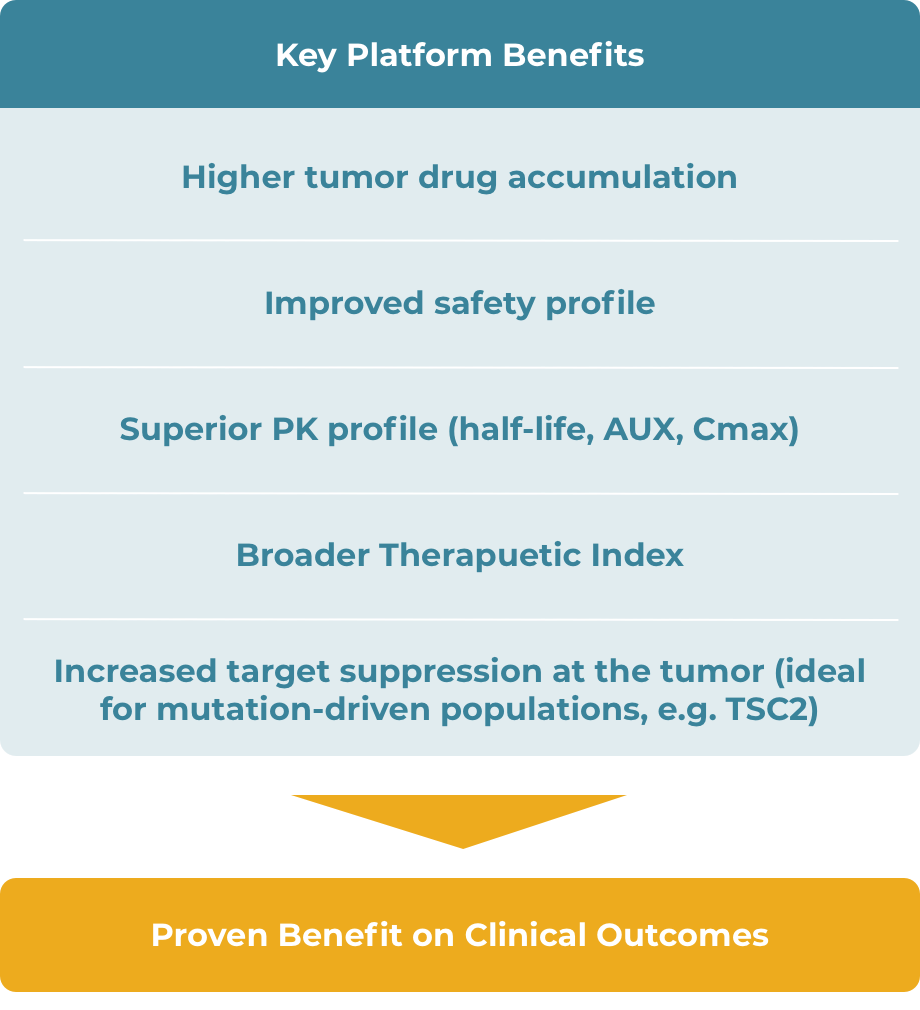
Tumor-Agnostic TSC1 and TSC2 Inactivating Mutations
Inactivating mutations in TSC1 and TSC2 drive mTOR pathway activation and tumor growth?
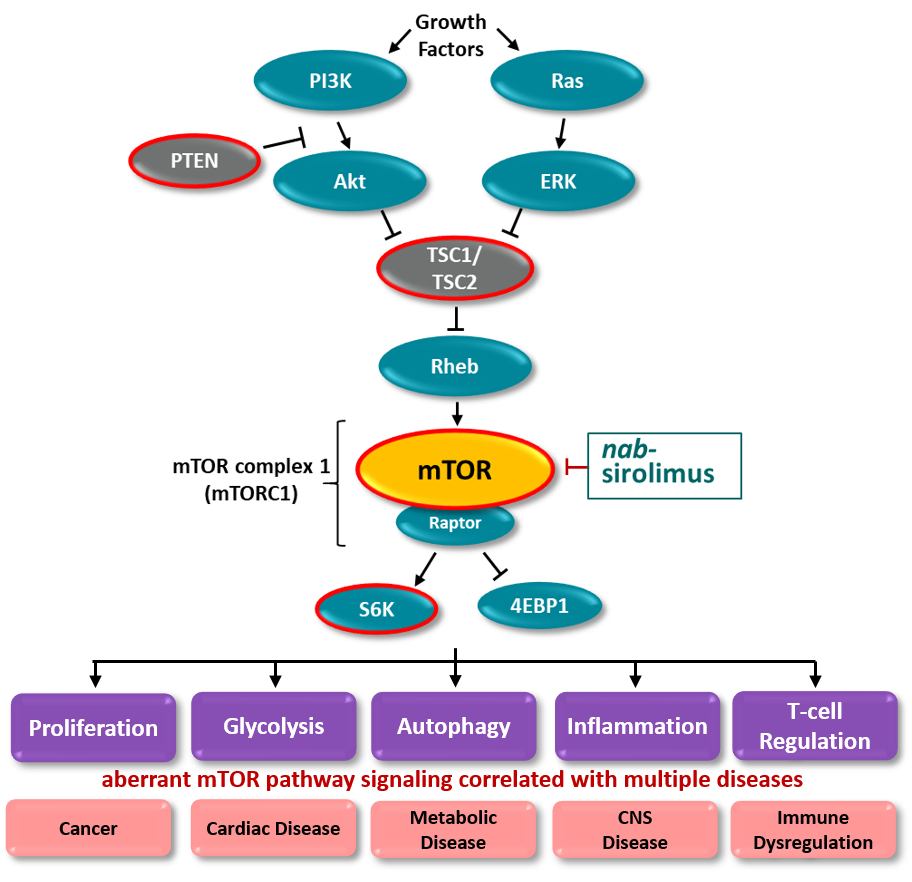
- TSC1 and TSC2 form a tumor suppressor complex that down regulate mTOR activity
- TSC1 and TSC2 mutations occur at a rate of approximately 1.4% and 1.8% across cancers respectively (Aadi internal estimate based on TCGA database analysis)
- No approved therapies for TSC1 and TSC2 mutant patients but numerous case reports with durable responses to mTOR inhibition
- Standard CLIA-certified NGS panels already capture TSC1 and TSC2 mutations
Increased probability of success for FYARROTM
- Striking clinical data in TSC1/TSC2 mutant patients in PEComa
- Encouraging signals in TSC1/TSC2 mutant non-PEComa patients in ongoing Expanded Access Program (details presented at ASCO 2021)
- Projected FDA approval for malignant PEComa by early 2022 significantly derisks large market opportunity in TSC1/TSC2 mutations
Tumor-Agnostic TSC1 & TSC2 Mutant Registrational Trial
- Registrational Phase 2 Study design and strategy discussed with FDA
- Planned 2H 2021 kickoff
- Similar approach to other precedent tumor agnostic registration studies
- Patient accrual at sites based on local NGS results and via diagnostic partnerships